
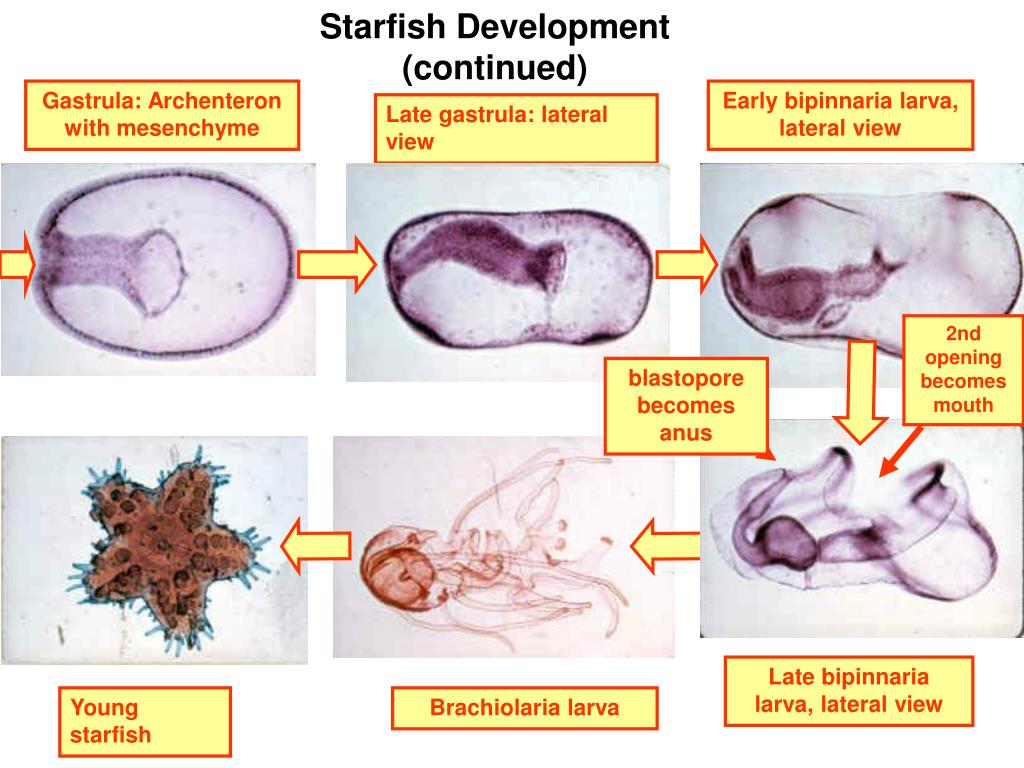
F) Comparison of blastopore compliance between early and late gastrulation stage embryos indicating decreased compliance over time.Ī) Schematic of gastrulating embryo at Stage 11 indicating regions of imaging (Scale bar represents 200μm).
Cantilevers used had spring stiffness values ranging from 0.002–0.004 N/m. Inset depicts representative force-displacement plot that is used to calculate spring stiffness at each point. E) Representative blastopore compliance over the course of gastrulation. Inset in D shows the loading regime of the cantilever acting on the blastopore (direction of black arrow). D) Schematic and D′) actual image of blastopore lip compliance experiment consisting of a fixed, stiff Kevlar cantilever (arrow) and a moveable compliant Kevlar cantilever (arrowhead). C) Force-time profiles from 3 different embryos subjected to force experiments, each displaying stall forces at approximately 4 hours. B) Schematic and B′) actual image of force experiment consisting of two fixed compliant cantilevers (arrowheads) b bottle cells on the dorsal lip, y the yolk plug. Scale bar in A represents 200μm.Ī) Protocol for force and stiffness measurements. Involution is occurring between −45 and 45 degrees at Stage 10 between −90 and 90 degrees at Stage 11 and all around the blastopore circumference at Stage 12.5. Regions outside the embryo and within the yolk plug are manually shaded gray. Cartesian plots were calculated within the ‘flat region’ to avoid distorted strain measurements from the embryo curvature. The cartesian plots (E,K,Q & F,L,R) represent the median (magenta line) and 75 and 25% quartiles of pixel-by-pixel strain at specific angles around the blastopore, binned every 1 degree. Radial and circumferential tissue strains are represented in polar (C,I,O & D,J,P) and cartesian (E,K,Q & F,L,R) coordinates during the beginning (A–F), middle (G–L) and end (M–R) of BC. Tissue net displacements (B,H,N) and strains were mapped using bUnwarpJ during BC (A,G,M). This figure shows analysis of a single embryo however results from 4 other embryos are consistent and are summarized in Figure S1. Strain and displacement analysis was done on stage 10 (A–F), stage 11 (G–L) and stage 12.5 (M–R) embryos. Cell shape and F-actin alignment analyses reveal different local mechanical environments in regions around the blastopore, which was reflected by the strain rate maps.īlastopore closure Cell polarity Cytoskeletal inhibitors Development Gastrulation Gastrulation mechanics Morphogenesis Nanoscale force measurement Tissue force production Tissue strain.Ĭopyright © 2014 Elsevier Inc. Interestingly F-actin was consistently oriented toward the blastopore lip in dorsal and lateral cells, but oriented parallel to the lip in ventral regions. The F-actin network is organized differently in each region with the highest percentage of alignment occurring in the lateral region. In contrast, cells lateral and ventral to the blastopore are less polarized and have tortuous cell boundaries. Cells dorsal to the blastopore, which are fated to become neural plate ectoderm, are polarized and have straight boundaries. Strain rate mapping of the dorsal, ventral and lateral epithelial cells proximal to the blastopore reveals changing patterns of strain rate throughout closure. During this time course, the embryo also stiffens 1.5 fold. We find that the embryo generates a ramping magnitude of force until it reaches a peak force on the order of 0.5μN. We describe the mechanics of blastopore closure at multiple scales and in different regions around the blastopore by characterizing large scale tissue deformations, cell level shape change and subcellular F-actin organization and by measuring tissue force production and structural stiffness of the blastopore during gastrulation. These forces are tuned to generate sustained blastopore closure throughout the course of gastrulation. Blastopore closure in the amphibian embryo involves large scale tissue reorganization driven by physical forces.


 0 kommentar(er)
0 kommentar(er)
